Gas laws
Gas laws: Overview
This topic covers concepts such as gas laws, Boyle's law, isotherm in graph, Charles' law, and graphs based on Charles' law.
Important Questions on Gas laws
A bubble of air is underwater at temperature and the pressure 1.5 bar. If the bubble rises to the surface where the temperature is and the pressure is 1.0 bar, what will happen to the volume of the bubble?
The correct value of the gas constant is close to:
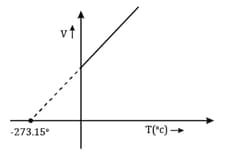
At constant pressure following graph represents
At STP, litre of a gas weighs . The vapour density of gas is -
Define isobars in the context of states of matter.
At STP, 5.6 litre of a gas weighs . The vapour density of gas is -
For comparing the same substance under different sets of conditions, Avogadro's law can be expressed as:
Explain why the number of atoms in a certain volume of hydrogen is twice the number of atoms in the same volume of helium at the same temperature and pressure.
A gas is heated from to at pressure. If the initial volume of the gas is , its final volume would be
Air expands on heating and hence its density _____. (increases/ decreases)
For a fixed amount of gas at constant temperature, volume of a gas is _____ proportional to the pressure of a gas.
The slope for an isochore plotted for a van der Waal's gas neglecting molecular attractions is equal to:
Normal temperature =_____ .
According to the Boyle's law, as the pressure increases volume
The absolute temperature value that corresponds to is
The values of Celsius scale can be converted to Kelvin scale by
On converting to Kelvin scale, the correct sequence of temperature will be
The Kelvin scale of temperature is always
Mention the relationship between the Celsius scale and the Kelvin scale of temperature.
Boyle's law equation : p =_____.
